We’ve all been there – the ever more precarious looking pile of filth by the sink continually grows, and eating dinner with a spatula out of the pan suddenly seems like a great idea. Newly dirtied utensils are carefully added to the pile, in the hope that the washing up can be put off for a few hours longer. Eventually, however, we must yield.
There is no doubt that washing up is a tedious task; however, have you ever stopped to wonder what is happening beneath the bubbles? Dirty dishes go in, wipe wipe wipe, and clean dishes emerge, as if by magic. If you tried to do this with just water it would be an almost futile effort, yet with the addition of a small drop of soapy gel, it becomes so much simpler. What, therefore, does the washing up liquid do to help de-grease and de-grime your utensils? The revelation will hopefully make your washing up time a little more interesting.
First we must think about what it is that is smeared all over your plates. Call it what you will, grime, grease, grunge, it all comes down to the fact that it is predominantly a fatty substance. It is fairly common knowledge that fats will not mix with water (try pouring some cooking oil in a cup of water to see this first hand), therefore, the problem lies in the fact that we are going to use water to try to remove a substance that will not mix with it.
There are three reasons why oil and water won’t mix. Firstly, the fat molecules are ‘hydrophobic’, a term that I also mentioned in my previous post about DNA (http://thatsinteresting.scienceblog.com/2013/01/22/dnawesome-2/), meaning that they won’t mix with water and they will instead group together in an attempt to escape it (take your cup of oil and water and agitate it, see how the oil forms into spheres as the molecules aggregate). Secondly, water has a very high surface tension; this is why it hurts so much to belly flop into a swimming pool! This is also why water on the edge of a tap will form into drops. A nice way to observe the surface tension of water is to carefully place a paper clip into a bowl of water (easiest to do using another paper clip to lower it is). The paper clip is made of steel, which sinks in water; however, the surface tension will keep the paper clip afloat. This will only work while the paper clip is dry, as the water on a wet paperclip will disrupt the surface tension, causing the paper clip to sink. Thirdly, oil and fats are less dense than water (0.9 g/cm3 compared to 1 g/cm3), so they will float on top instead of mixing with it. This is also why obese people float more easily in the swimming pool; it is nothing to do with weight; it is all about density. The combination of the high density and surface tension of water, and the hydrophobicity of the fat, means that oils and fats will sit on top of the water and never mix with it.
Therefore, in order to wash our dirty dishes successfully, we must overcome these obstacles. There is not much we can do about the densities, as these are physical properties of the fat and water, but with the addition of washing up liquid we can affect the surface tension and hydrophobicity.
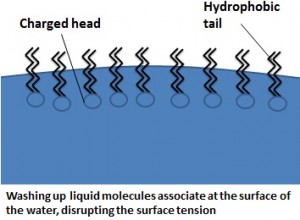
To understand how, we first need to consider the properties of the washing up liquid. Other than some vivid colouring agents and pleasant smells, washing up liquid contains millions of molecules that are said to be ‘amphipathic’ .This means that they are composed of a ‘head’ and ‘tail’ domain, the head is charged (and therefore hydrophilic) and the tail is hydrophobic. It is this interesting property that allows the washing up liquid to work so effectively.
So, firstly, how does this affect the surface tension? Washing up liquid is a ‘surfactant’, meaning that it will lower the surface tension of water. The hydrophilic heads will dissolve in water but the tails want to remain dry, so the molecules will group together at the surface of the water, with the tails sticking out into the air. The charged head groups interfere with the bonds between the surface water molecules that maintain the high surface tension, and thus it is released. A great, simple experiment to show this is to take your bowl of water with a paper clip floating on the surface and add a tiny drop of washing up liquid to the side of the bowl. As soon as it contacts the water the paper clip will drop to the bottom! The other way that the surface tension of water is lowered during washing up is through agitation of the water by the person who is doing it.
That’s the first issue solved, but what about the hydrophobicity of the fat? Even though it will now mix more readily with the water it still won’t dissolve in it, which would make it easy to remove with a sponge. Again is it the amphipathic washing up liquid molecules that overcome this. When mixed into the water, the hydrophobic tails of the washing up liquid will all group together into a ball, with the hydrophilic heads pointing out towards the water, forming a ‘micelle’ (my-cell). If, before they group together, these hydrophobic tails encounter a hydrophobic fat molecule in the water, they will bunch around it in the same way, surrounding it with charged head groups, allowing it to dissolve in water. Because the outside surface of all of these balls of fat and washing up liquid are slightly charged, they will also repel each other, preventing the fat molecules form clumping together again. This is known as emulsification.
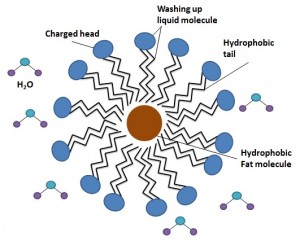
Now the greasy fat has been happily mixed with, and dissolved in, water and it can be much more easily scrubbed off with a sponge. It is just one big chemical reaction! The use of hot water simply speeds up this reaction, making the washing up quicker and easier. Also it shows that there is some science behind leaving the most stubborn pans in the sink overnight to soak. This is not simply a way of putting the job off until tomorrow, rather it gives the washing up liquid molecules much more time to surround and dissolve the fats, meaning they will be easier to dislodge later.
So, washing up is actually a lot more interesting than it seems, with some very important processes taking place deep beneath the bubbles. It is just one example of how science helps to improve your everyday life and I hope this allows you to enjoy the washing up a little more next time you do it.