Summer is upon us. Or, if you are British, it may already have passed. During the past few weeks of glorious, if unexpected, sunshine in the UK and Europe, sales of sun cream (or sun screen for the Americans) have soared.
But how does sun cream protect your skin from the Sun’s unrelenting rays? How can this unassuming cream provide a barrier against such a powerful adversary? These are clearly burning questions.
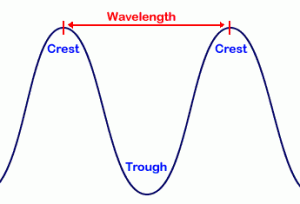
In his famous double-slit experiment, the physicist Thomas Young proved that light has the properties of a wave. The standard measurement used to describe the properties of a wave is the wavelength, that is, the distance between two matching points, such as peaks or troughs, as shown in the picture. Visible light has a wavelength of 400-700 nanometers (1 nanometer (nm) = one billionth of a meter), and at a slightly lower wavelength, ultraviolet (UV) light has a wavelength of 10-400 nm, both of which are emitted by our Sun. The vast majority of the emitted UV energy (>97%) is absorbed by the ozone layer, the protective part of our atmosphere, without which, we would be cooked. The UV energy that is able to seep through the ozone layer is split into 2 categories, UV-A (320-400 nm) and UV-B (290-320 nm). It is the latter of these, UV-B, which is responsible for tanning and eventual sunburn. UV-A, on the other hand, is able to pierce deeper into the skin, causing the skin to age and wrinkle, eventually leading to cancer. On an interesting side-note, UV-B cannot travel through glass, hence you cannot burn through a window; however, UV-A waves can, so can still cause damage when you are inside.
In order to avoid sunburn, a barrier needs to be formed between your skin and the damaging UV rays. Sun cream employs two groups of molecules to do this, some that reflect UV rays, and some that absorb them, both of which prevent them from reaching the skin.
Zinc oxide and titanium dioxide are the two most common substances used to reflect UV rays. Their ability to reflect both UV and visible light is the reason why people wearing higher factor sun cream appear to ‘glow’ more in flash photography. White zinc oxide cream also is a common feature with surfers and cricketers (mainly as a distinctive fashion statement), and can be seen adorning the lips of some of the England players, while they are busy retaining the Ashes this summer!
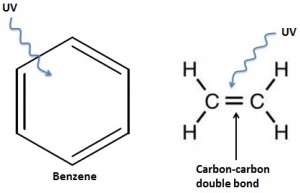
Several complicated sounding molecules are used to absorb the UV rays such as para-aminobenzoic acid, Cinnamates, and Benzophenones. The common features of these are the presence of a ring of 6 carbon atoms, called benzene, and also several carbon-carbon double bonds. Most importantly, both benzene and carbon-carbon double bonds have a pool of shared electrons. These float around between the atoms and it is the sharing of these electrons that forms the strong bond between them. It is this pool of shared electrons that is able to absorb the UV energy, and release it as heat, meaning that it doesn’t reach your skin.
One final important point is that the sun protection factor (spf) on sun cream only relates to the protection provided against UV-B rays, as this is what causes sun burn. It is important to use a sun cream that gives a broad range of protection, against both UV-A and UV-B.
So it is the combined effect of molecules that both reflect and absorb UV radiation that allows sun cream to act so effectively. As it is such a ubiquitous commodity, the fascinating science behind sun cream is largely ignored. Its ability to protect us so effectively from the dangerous UV radiation of the Sun, coupled with its ease of application and almost non-existent side effects, makes it, in my opinion, one of the best inventions around today… although this may be just because I am pale and pasty.

That was great Ben! Never knew the difference between UV-A and UV-B before, something to look out for in future suncream purchases!
This is really interesting Ben, I had completely forgotten that UVA passes through glass! I rarely go out without my factor 30 so am feeling smug now!
Wow!! Thanks for the information. I now have a better understanding of UVs and how to protect my skin.